Publications/Presentations referencing FreeThink’s work
L.Dai, J.Davis, K.Nagapudi, P.Mantik, K.Zhang, J.Pellett, and B.Wei
“Predicting Long-Term Stability of an Oral Delivered Antibody Drug Product with Accelerated Stability Assessment Program Modeling”
Molecular Pharmaceutics
December 2023
D.Kumar Singh and S.Singh
“Drug Substance/Product Quality Analysis (Quality Assessment)”
The Quintessence of Basic and Clinical Research and Scientific Publishing
October 2023
J.Fine, P.Wijewardhane, S.Dawood Beer Mohideen, K.Smith, J.Bothe, Y.Krishnamachari, A.Andrews, Y.Liu, G.Chopra
“Learning Relationships Between Chemical and Physical Stability for Peptide Drug Development”
Pharmaceutical Research
March 2023
E.de Pablo, P.O’Connell, R.Fernández-García, S.Marchand, A.Chauzy, F.Tewes, M.Dea-Ayuela, D.Kumar, F.Bolás, M.Ballesteros, J.Torrado, A.Healy, D.Serrano
“Targeting lung macrophages for fungal and parasitic pulmonary infections with innovative amphotericin B dry powder inhalers”
International Journal of Pharmaceutics
March 2023
P.Legrand, S.Dufaÿ, N.Mignet, P.Houzé, R.Gahoual
“Modeling study of long-term stability of the monoclonal antibody infliximab and biosimilars using liquid-chromatography-tandem mass spectrometry and size-exclusion chromatography-multi-angle light scattering“
Analytical and Bioanalytical Chemistry
January 2023
N. Zhao “Discussion on the Technical Requirements and Methods for In-use Stability Testing of Drug Products”
Chinese Journal of Clinical Pharmacology
National Drug Administration, Drug Evaluation Center, Beijing, China
January 2022
S. Buchanan and S. Konagurthu “Accelerated stability assessment for an oral N-type calcium channel blocker”
Thermo Fisher Scientific
Presented at AAPS PharmSci 360, October-November 2020
M. McMahon, et al. “Utilization of risk-based predictive stability within regulatory submissions; industry’s experience”
SpringerOpen: AAPS Open
May 2020
F. Bidet “Rethinking Dry Powder Inhaler Packaging: New Solutions to Old Challenges”
Aptar CSP Technologies
Presented at Pharmapack EU, Paris, France, February 2020
T. Rawat, et al. “Accelerated Stability Modeling of Drug Products using ASAPprime®”
Perrigo Company
Presented at AAPS PharmSci 360, San Antonio, Texas, November 2019
F. Qiu, et al. “Accelerated Predictive Stability: Fundamentals and Pharmaceutical Industry Practices”
This book discusses many aspects of ASAP, with case studies.
© 2018

“Accelerated stability assessment program: a speedy and scientific approach for shelf life setting of drug product—feasibility study of Accelerated Stability Assessment Program”
IMP Quality Project, Pharmaceutical Expert Committee, Quality and Technology Committee, Japan Pharmaceutical Manufacturers Association
Pharmaceutical Technology Japan: Volume 34, Issue 5
2018
H. E. Williams, et al. “A comparison of drug substance predicted chemical stability with ICH compliant stability studies”
Drug Development and Industrial Pharmacy
Published online November 2018
G. Scrivens “Predicting the Long-Term Dissolution Performance of an Immediate-Release Tablet using Accelerated Stability Studies”
Pfizer
Presented at Science of Stability Conference, Boston, Massachusetts, October 2018
S. Thielges “ASAP for Parenteral Formulation Development – Solutions and Lyophilisates”
Novartis
Presented at Science of Stability Conference, Boston, Massachusetts, October 2018
D. Stephens, et al. “Risk-Based Predictive Stability for Pharmaceutical Development–A Proposed Regulatory Template”
PharmTech: Pharmaceutical Technology: Volume 42, Issue 8
August 2018
H. Williams, et al. “Risk-Based Predictive Stability–An Industry Perspective”
PharmTech: Pharmaceutical Technology: Volume 41, Issue 3
March 2017
M. Knopp “Stability Modeling and Package Selection”
Tablets & Capsules: CSC Publishing Inc.
May 2016
H. Li, et al. “Prediction of the Changes in Drug Dissolution from an Immediate-Release Tablet Containing Two Active Pharmaceutical Ingredients Using an Accelerated Stability Assessment Program (ASAPprime®)”
SpringerOpen: AAPS Open
2016
H. Forcinio “Building a Barrier in Solid-Dosage Drug Packaging”
PharmTech: Pharmaceutical Technology: Volume 39, Issue 1
January 2015
S. Thielges “ASAP Concept and Case Studies”
Janssen Pharmaceutica
Presented in London, England, March 2013
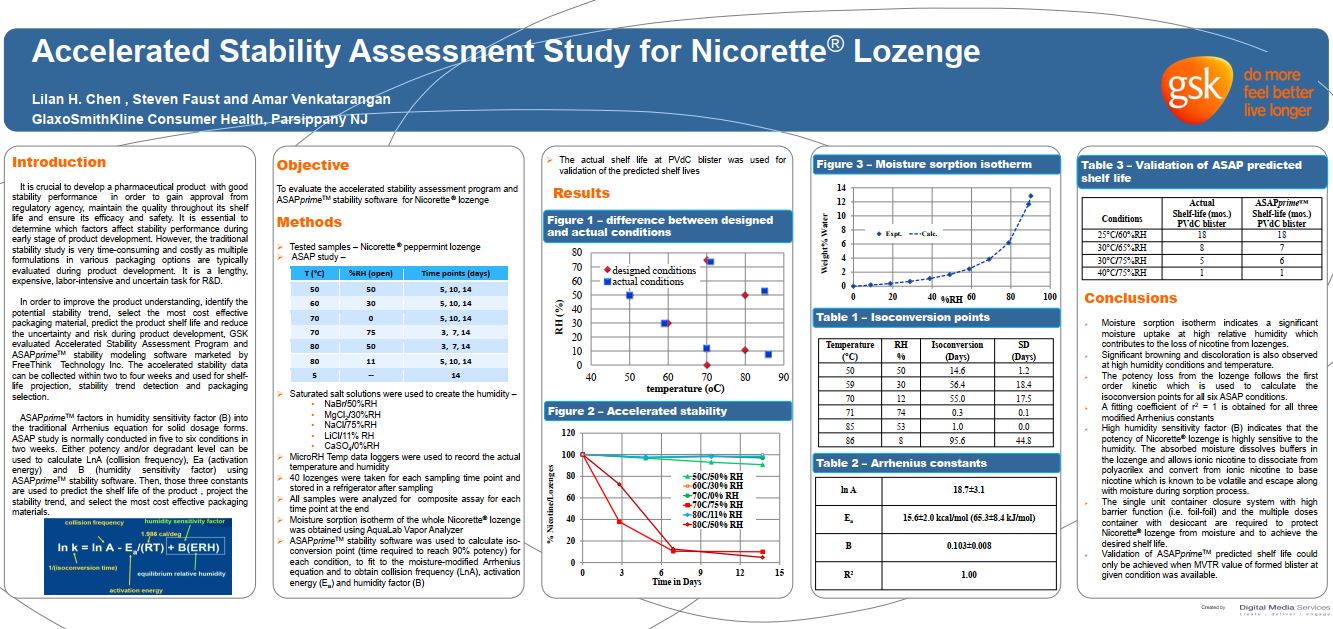
L. Chen, et al. “Accelerated Stability Assessment Study for Nicorette® Lozenge”
GlaxoSmithKline Consumer Health
Presented at AAPS Annual Meeting and Exposition, San Antonio, Texas, November 2013