ASAPprime® Stability Testing Software
ASAPprime®: The industry standard for accelerated stability software modeling
-
Licensed by the majority of large pharmaceutical companies around the world.
-
Accurately determines the shelf-life of products in very short time periods.
-
Successful with liquids, gels and solids with many types of stability-indicating factors.
-
Determines packaging without the need for package screening studies.
The details:
ASAPprime® is a stability software package based on the Accelerated Stability Assessment Program (ASAP) and uses experimental data modeling to accurately determine the shelf-life of products in very short time periods.
ASAPprime® includes two parts: ASAPdesign™, and the main user interface.
ASAPdesign™ leads the user through questions aimed to provide an experimental plan for an ASAP study of a specific product. This plan is based on product knowledge, design space limitations of temperature and relative humidity (RH), data precision required, time available, and the number of samples chosen to be analyzed.
Once the study is carried out, the results are input into the main program to build a mathematical model of the product behavior. The modeling uses a statistical and scientific fitting process to provide the user with the probability that a product will remain within its stability-indicating specification limits at the designated shelf-life time, based on the selected storage conditions and packaging options.
ASAPprime® differs from forced degradation since it is designed for predictive shelf-life determinations (modeling) rather than as a test of analytical method appropriateness.
Questions? Contact a Stability Software Expert
ASAPprime®: A Faster, More Scientifically Accurate Paradigm for Stability
Traditional stability studies
Samples are placed in stability chambers (usually in packaging) with measurements occurring at various, fixed time points. Changes such as the growth of impurities, loss of active ingredients or other stability-indicating factors are monitored, and rates extrapolated accordingly. When higher temperatures/RH conditions are used, there is often significantly more degradation than at the lower, less harsh conditions.
ASAPprime®
The product is exposed, without package protection, to conditions and time points designed to make the product hit, but not greatly exceed, its specification limit. ASAPprime® determines the “time to fail” (isoconversion times) at a range of conditions; then uses these data to model the long-term behavior.
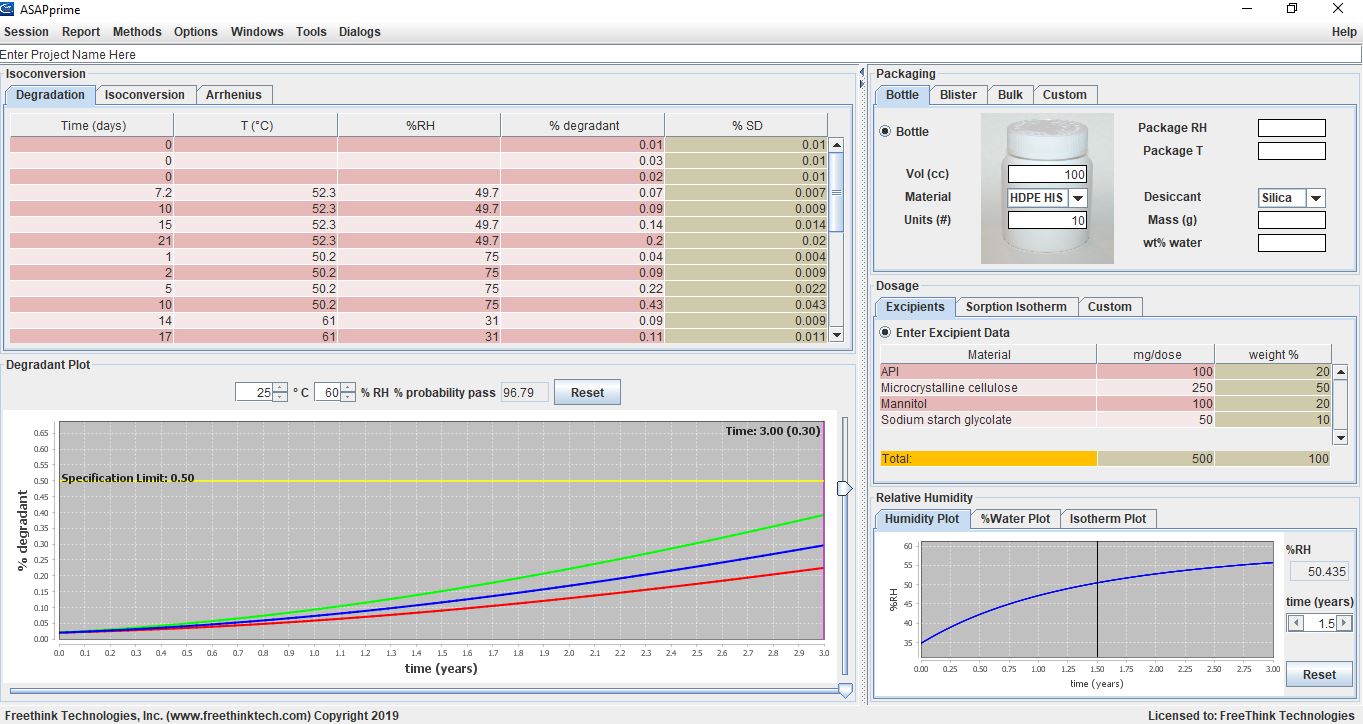
Screen shot of an ASAPprime® model of the stability behavior of a product showing that with the indicated packaging, the product has a high probability of passing (staying below the degradant specification limit) for three years under Zone 2 conditions (25°C/60%RH).
ASAPdesign™ – Statistically Design an ASAP Study to Provide Good Shelf-life Modeling.
ASAPdesign™ is a subroutine within ASAPprime® that designs an optimal study for data modeling.
ASAPdesign™ first considers the precision of the measurements needed to determine the failure point and thereby establishes the number of repeats needed to power the study adequately.
The scientist determines how many analyses will be used (typically 10-30) and how long the study will last (typically 2-6 weeks). The program then populates an optimized design with respect to repeats, conditions (temp., RH) and time points. The program limits the design space based on any phase boundaries.
To hit its specification limit at as many conditions as possible, the program incorporates available prior knowledge about the product to establish appropriate conditions and time points.
When an ASAPprime® study is carried out in shorter overall times, there will be greater extrapolation to the long-term conditions with correspondingly larger error bars.
When the design requires RH control, ASAPdesign™ provides information about the appropriate saturated salts to use at each condition. These studies are carried out in individual jars containing vials of salt slurries to control the RH in “mini-chambers.”
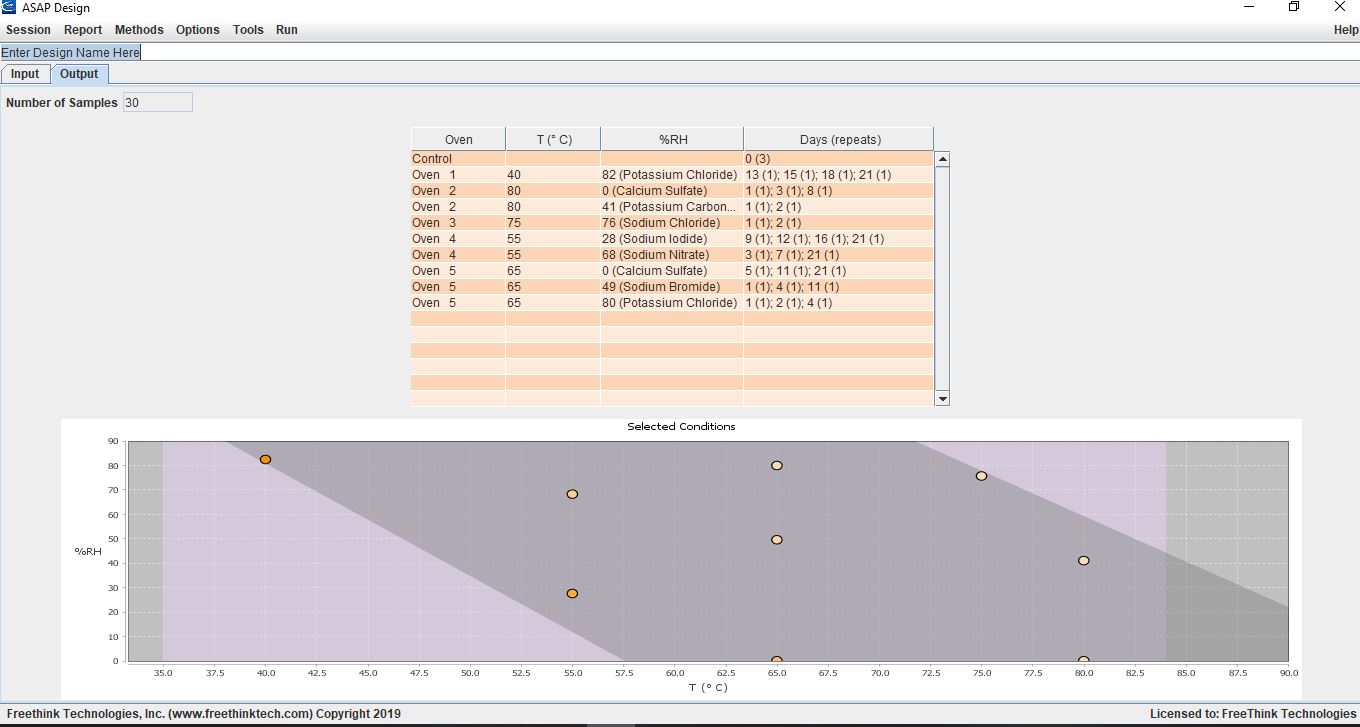
Screen shot from ASAPdesign™ showing an example design space and timepoints for carrying out an ASAPprime® study of a tablet.
